Oral antiplatelet agents are central to the management of patients following acute coronary syndrome (ACS). Evidence-based practice guidelines from the American Heart Association (AHA) and American College of Cardiology (ACC) recommend long-term antiplatelet therapy to prevent recurrent ischemic events in these patients; generally aspirin is recommended indefinitely, plus a thienopyridine for at least 1 year. Despite these guideline recommendations, evidence from clinical practice suggests that antiplatelet agents are being substantially underutilized. Often antiplatelet therapy is not prescribed in eligible patients at the time of hospital discharge or patients fail to submit or collect their prescriptions. Even when dual antiplatelet therapy (DAPT) is commenced, patients may prematurely discontinue therapy, resulting in an increased risk for further thromboembolic complications. A variety of factors contribute to this discontinuation, including not being counseled on the importance of continuation of therapy, nuisance bleeding, and cost, and because of the recommendation of the patient's family physician, dentist, or other healthcare provider who does not have a complete understanding of the risk:benefit ratio of this therapy. Our expert panel of pharmacists discusses existing and novel antiplatelet agents and strategies to improve patient adherence to long-term DAPT.
Slide 1.
John R. Fanikos, RPh, MBA: Hello. I'm John Fanikos, Director of Pharmacy at Brigham and Women's Hospital and Assistant Professor of Clinical Pharmacy Practice at Northeastern University and the Massachusetts College of Pharmacy. Welcome to our program, Pharmacists' Role in ACS Management: Translating Knowledge to Clinical Practice.
Joining me today at the American Society of Health-System Pharmacists meeting in Anaheim, California, is Sarah Spinler, Professor of Clinical Pharmacy and Clinical Residency Program Coordinator at the Philadelphia College of Pharmacy, University of the Sciences in Philadelphia, Pennsylvania. Also joining us is Paul Dobesh, Associate Professor of Pharmacy Practice at the College of Pharmacy at the University of Nebraska Medical Center in Omaha. Welcome.
Paul, I'll start with you. We've had clopidogrel as an antiplatelet agent for many, many years now. Can you give us the landscape of newer agents that are available, pending approval for practitioners, relative to antiplatelet therapy?
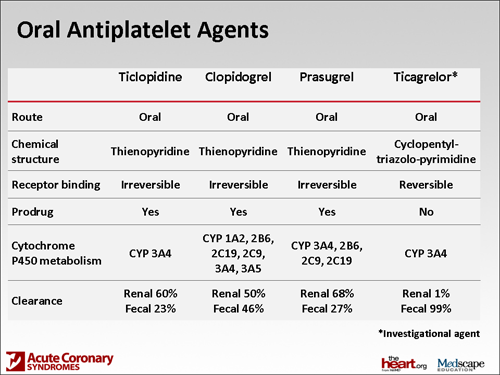
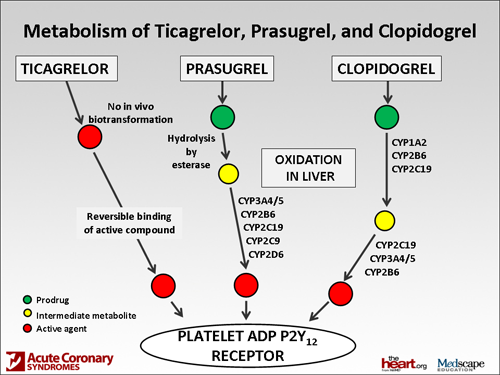
Paul P. Dobesh, PharmD: You're right, John. We've had clopidogrel around for well over a decade now. Actually, it's an exciting time because just within the last year, we have a new agent called prasugrel. Prasugrel and clopidogrel are in the same class of antiplatelet agents: they're both thienopyridines. They both need to be converted to active compounds, so there are a lot of similarities between the drugs.
Slide 2.
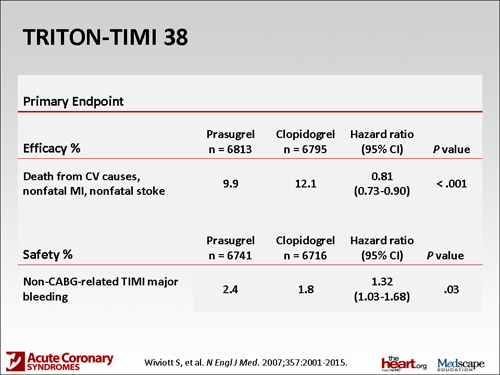
The 2 drugs have been compared together in a head-to-head trial called the TRITON-TIMI 38(Trial to assess Improvement in Therapeutic Outcomes by optimizing platelet Inhibition with prasugrel Thrombolysis In Myocardial Infarction 38). In that study, prasugrel actually provided a significant reduction in cardiovascular outcomes. There was a slight increased risk for bleeding that was statistically significant. It was one of those things where they couldn't have their cake and eat it too: better outcomes but a little more bleeding.
The reason for that is that if you look at the molecule of prasugrel, it's been shown to provide more potent and much more consistent antiplatelet effect. One of the things we've known about clopidogrel is that there is a large variability in effect in ability to inhibit the platelet. We don't seem to see that as much with prasugrel. If you inhibit the platelet more, you reduce outcomes, but if you inhibit the platelet more, you also have an increased risk for bleeding. Some of that bleeding was pretty severe; life-threatening bleeding was significantly higher and fatal bleeding was significantly higher. Clearly, some things we'd have to think about if we were going to select a patient to get prasugrel.
In fact, 3 groups of patients were definitely found to be at higher risk for bleeding. One is patients who had a history of a transient ischemic attack or a stroke. That is actually a boxed warning -- that those patients should not get prasugrel; it's a contraindication. Two other groups of patients are those over the age of 75 or those who weigh less than 60 kg. Those patients also are at an increased risk for bleeding. There are not absolute contraindications there but still some real cautions to worry about. We have prasugrel, a drug that provides more potent antiplatelet activity. There are some benefits there but also some safety concerns. Clearly, it's something that advances where we've been with antiplatelet therapy.
Would you like to talk about ticagrelor?
Slide 3.
Sarah A Spinler, PharmD: Ticagrelor is a new agent that was recently approved in Europe. Approval in the United States should be expected shortly. Ticagrelor is a non-thienopyridine antiplatelet agent. It differs significantly from prasugrel in that it's directly active, so it does not have to go through as many metabolic steps and does not have as many significant drug-drug interactions as does clopidogrel. Prasugrel is still a prodrug, as is clopidogrel, and ticagrelor is not.
A difference, though, in the pharmacokinetics is that it does have a shorter half-life such that you have to administer it twice a day, compared with prasugrel once a day and clopidogrel once a day. Also, if you look at some of the significant drug-drug interactions, it's not devoid of those. It is likely to be contraindicated in patients who are receiving strong CYP3A4 inhibitors as well as inducers and for patients who, for instance, are on cyclosporine; which are substrates of 3A4 but have a very narrow therapeutic index, and that's in accordance with the PLATO (PLATelet inhibition and patient Outcomes) trial.
Slide 4.
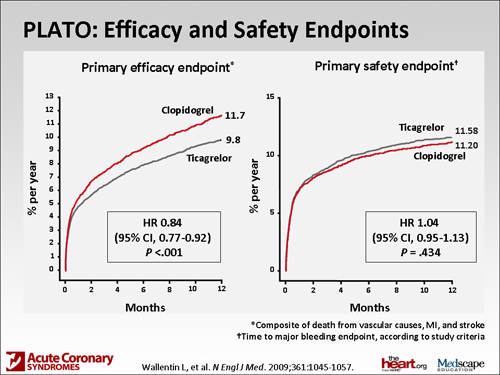
Ticagrelor and prasugrel, although both newer agents, have not been directly compared in a head-to-head trial. Ticagrelor was compared with clopidogrel, and as Paul mentioned, similar to the prasugrel trial, a significant reduction in cardiovascular death, myocardial infarction (MI), or stroke was demonstrated. It was slightly different in the definition of major bleeding in that study. They had their own PLATO-defined major bleeding, which was not significantly increased with ticagrelor. However, the non- coronary artery bypass graft (CABG) major bleeding still was elevated in the patients receiving ticagrelor, so it's similar to having more potent platelet inhibition, which ticagrelor does have. Compared with clopidogrel, there was an increased risk for non-CABG major bleeding.
Another significant endpoint that was found in that trial that was a little bit different from the TRITON trial was that there was a significant reduction in cardiovascular mortality. I think that's what many cardiologists are excited about with that particular drug. Again, that comes with the price of taking the drug twice a day, compared with once a day with clopidogrel and prasugrel.
Mr. Fanikos: What I'm hearing is that there are clearly some advantages on the efficacy side. Neither of you mentioned in-stent thrombosis, which is a worry, so did we have better outcomes in that setting? Because I know the cardiologists all worry about that as well as making sure that patients continue their therapy.
Slide 5.
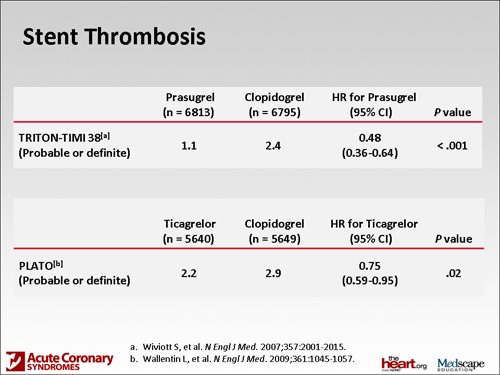
Dr. Dobesh: Yes; I know that in both studies there was a significant reduction in stent thrombosis. We bring up a really important point, which is that in order for these drugs to work, we can talk about these significant benefits, but if patients don't take their medications, they're going to have problems. It really doesn't matter whether it's clopidogrel, prasugrel, or ticagrelor; patient adherence is going to be a critical issue because these are drugs that aren't inexpensive that need to be taken for a significant amount of time, usually at least a year, depending upon the clinical situation. I think that makes patient adherence, with all of these medications, a critical issue.
Mr. Fanikos: That's where the role of the pharmacist becomes critically important in translating what is played out in clinical efficacy in these trials and putting them into practice in both the hospital setting, where the drugs are most frequently initiated, and then the community setting. We know from several combined trials now that many times there are delays in patients filling their prescription. I know data from at least 1 trial suggest that a significant number of patients never actually get to the pharmacy and never have their prescriptions filled. In the ones who did not, at least in the setting of antiplatelet therapy, there was a higher incidence of death from MI.
So what are the important things, as a hospital pharmacist, that pharmacists can focus on in improving what happens when their patients are ready for discharge?
Dr. Spinler: I think there's a huge focus on inpatient management of both MI and heart failure to prevent rehospitalization. Significant stent thrombosis is a typical example of a hospital readmission that you would like to prevent, especially with the newer agents where maybe you're not quite sure that a patient's insurance covers it, or you're concerned about a patient's copay -- that the patient can afford it. We work very closely with our discharge planners at our institution; they can actually contact the pharmacy that the patient will be getting the prescription from to see what the patient's copay is. We definitely communicate that with the patients before they leave the hospital to make sure that they are agreeable to that before we send the patient home on that particular medication. That's one of the ways that we work with that at the time of hospital discharge.
Dr. Dobesh: We do something that's very similar to that -- working with our discharge planners. All of our pharmacists are involved in the discharge counseling of medications and that's not consistent (across different institutions) . There are a number of hospitals where nursing does discharge counseling, or it could fall in different hands. Our hospital is one of the ones in which pharmacists have taken that on. We thought that if someone is going to be educating someone about medications, it probably needs to be the pharmacist. That's part of the job that we take on. Then it really transitions to the community pharmacy setting to make sure that there is restating of the importance of these medications, because usually they're not going home just on 1 of these 3 drugs; it could be 6 or 8 new drugs, depending on what their history is. Therefore, I think it's going to be very important that they have an understanding of why they're taking all of these medications, as well as the importance of staying on those medications.
Slide 6.
Mr. Fanikos: There was a recent trial published in Annals of Internal Medicine that said that as many as 3% of prescriptions are abandoned at the local pharmacy, so even in the community setting the pharmacist has the opportunity to intervene when those prescriptions aren't picked up, especially for antiplatelet therapy, and make sure that those medications get into the hands of the patients.
Paul, I know you've done a lot of work [in this area]. These medications are typically lifelong. What do we do with chronic medications and patient adherence?
Dr. Dobesh: You had mentioned some studies that have looked at the importance of patient adherence and connected it to outcomes. There was a paper in Circulation [1] not that long ago that looked at patients who got clopidogrel: those who only had gotten the first month of their clopidogrel filled vs those staying on it for the full year. Those who stayed on it for the full year who were adherent -- because they're all supposed to be put on it for the first year -- had significant reductions in things like stent thrombosis and cardiovascular outcomes. So once again, more data supporting that. We have to think about ways that we can help our patients better adhere to their medications. I know that it's easier for me to sit here and say it than to figure out how to do it.
One thing we looked at in a study we did was the number of times a day the patients take a medication. Sarah had mentioned the fact that ticagrelor is a twice-a-day medication. The other ones are once-a-day. There are conflicting data in this. Does it matter whether it's a once- vs a twice-a-day drug? Some data say no, it probably doesn't. The structure of those studies is that a lot of them are looking at electronic filling and things like that, and the patients know they're being investigated, so there may not be a real separation there.
Slide 7.
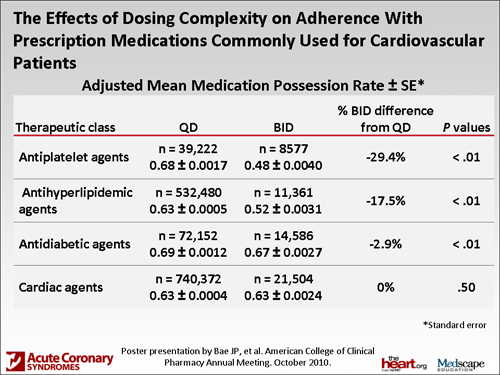
We actually looked at market scan data -- one of the largest evaluations of cardiovascular medications and adherence -- with once- vs twice-a-day medications. We had 1.4 million claims in our database. When we evaluated that, there was significantly better adherence using the medication possession ratio, which basically looks at refill data. We found a significantly better adherence with the patients on once-a-day vs twice-a-day, but the problem is that it was 63% vs 60%. It was really, really poor all the way around.
With cardiovascular medications, we had broken them up into 4 different categories, and one of the specific categories that we looked at was antiplatelet drugs. Interestingly enough, that was the area that had the greatest dispersion or the greatest difference between once- vs twice-a-day medications. For those who were on once-a-day, the adherence rate was 71%, vs 51% for those on twice-a-day. That, like I said, was the biggest dispersion. That's a 28% relative and a 20% absolute difference in adherence. However, there are data from the Response to Ticagrelor in Clopidogrel Nonresponders and Responders and Effect of Switching Therapies (RESPOND) trial with ticagrelor showing that even if you did miss a single dose of ticagrelor, because it provides such potent antiplatelet effect up front -- better than clopidogrel -- instead of taking it every 12 hours, you take it at every 24 hours. You actually end up with about the same amount of platelet inhibition as you would with a standard dose of clopidogrel. I would never educate my patients that it could be taken once a day, and it's okay to miss a dose, but at least I don't think we're going to have catastrophic events in patients missing single doses. I think it really comes down to long-term adherence. It's really almost an issue of patients being persistent. Adherence vs persistence is a slightly different evaluation.
Mr. Fanikos: So we look for strategies, really, for patients to take their medication around a daily event -- the alarm clock, breakfast, brushing your teeth, a shower -- to be consistent as they move forward and find ways of scheduling their medications on a day-to-day basis.
Dr. Spinler: Most of the medications can be taken together and they don't have to be separated. There are not a lot of drug-drug interactions related to administering them at the same time. Most of our patients who have come in with indications for these antiplatelet drugs, such as MI or a percutaneous coronary intervention (PCI), usually are on a twice-daily regimen -- some group of drugs in the morning or a group of drugs in the evening. It's not just once a day. We try and move everything to once a day if possible.
Slide 8.
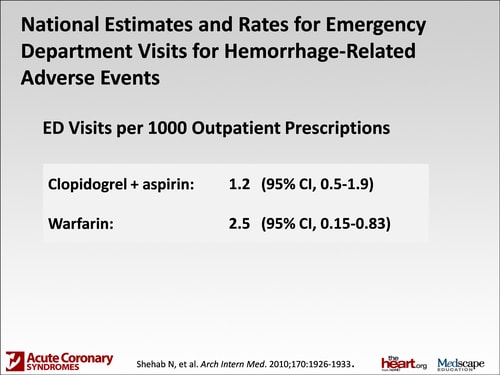
Mr. Fanikos: I want to switch gears and talk about side effects. You've talked about major bleeding episodes and how severe they are, but I wanted to talk a little bit about probably what is considered minor bleeding and nuisance bleeding. There was a trial that came out that looked at emergency department visits. It showed that there's 1.2 emergency department visits for every 1000 prescriptions that are filled for clopidogrel related to bleeding events. Warfarin is far higher at 2.5, so clearly we have more events with warfarin, but patients still show up at the emergency department with bleeding episodes from cuts or nose bleeding. What do we tell patients about episodes like that and either continuing or stopping their medication?
Slide 9.
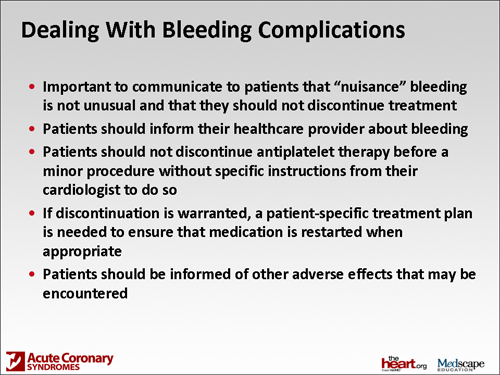
Dr. Dobesh: I think that I would stress to those patients that this is a risk. They should be aware, and that way they don't get frightened if they get a cut. If they're working in the yard and they get a cut and have a really hard time getting it to stop bleeding, or if they have a nosebleed or if they get a really big bruise, they're surprised by that. I think that's part of the basic initial education. You're going to be on a drug, whether it be clopidogrel, ticagrelor, or prasugrel with aspirin, so dual antiplatelet therapy. That should be part of the basic education, that (1) when that happens, not to be fearful, and (2) that it's part of taking the medication. They should let their physician know, let their pharmacist know. But they really need to stay on the medication.
In that same paper, 11% of patients had to stop their dual antiplatelet therapy. That, once again, could be a really big problem -- if they're not being persistent with their medication. They need to understand that this is something that can happen but they still need the medication. Let us, as the healthcare providers, know about this, but don't just stop your medications because much worse things can happen than a nuisance bleed if they stop their medications.
Mr. Fanikos: Sarah, you've weighed in on national guidelines. What about patients who are going for dental procedures and surgical procedures? What do we tell them?
Dr. Spinler: As we're going to a strategy of a medical home, the cardiologist needs to serve as the director of whether these agents should be stopped for procedures. I think we should communicate that to the patient. It doesn't matter whether they see a dentist or a surgeon -- usually it's the patient's primary cardiologist or it may even be the interventional cardiologist who put the stent in; the patients will leave with a definite plan as to how long they should be taking the dual antiplatelet therapy.
When they come with that original plan, I would urge community pharmacists to figure out what that plan is, so at least the community pharmacist will know as well. I always think that they should communicate that, or reinforce that, every time they see the patient. Sometimes we don't think about it -- "Oh, this patient has been on this therapy for a long time," -- but it's very good to reinforce the benefits of why they're taking it. It may not be as important for stent thrombosis, necessarily, as it is early on. But it certainly can be a problem with late stent thrombosis, if the patient has a drug-eluting stent. I think it's important to reinforce that. I think it's important, as far as the bleeding events go, to also reinforce the benefits. If the patient is completely black and blue and it's been 13 months since they've had their stent placed, that patient may be told by their cardiologist that they could discontinue it, but I think (it is important to put that patient) with one person who has responsibility as a gatekeeper, so to speak. Typically, the guidelines will suggest that in terms of looking at management strategies.
There are some Canadian guidelines now that have a lot more guidance and suggest that there are specific procedures that you don't discontinue clopidogrel for. But we don't have such guidelines for dual antiplatelet therapy. We don't have such guidelines in the United States. It's similar to what we've been trying to do with warfarin in terms of looking at how to judge stopping warfarin or how long to stop warfarin because of a certain procedure. But we don't have such specific guidelines. It's often a judgment call. It's made between how far the patient has been away from the initial stent placement and what the patient's risk is of bleeding from the particular procedure. With most dental procedures, you would not have to discontinue it. Again, the decision should be made in conjunction between the dentist and the cardiologist.
Dr. Dobesh: I would agree completely with Sarah. The pressure needs to be taken off of the patient. If another practitioner [tells the patient to stop the medication, then] the patient really just has to say, "You need to speak with my cardiologist” and let them handle it on the side, and not get in the middle if they say: “They want me to stop.” I agree with you completely. The cardiologist really has to be the gatekeeper here, and the patient knows that upfront and feels some security about that upfront.
Dr. Spinler: But I also think the patient should come back and say, "When should I resume it if it is being stopped for the procedure?"
Dr. Dobesh: Yes.
Mr. Fanikos: That needs to be communicated by the practitioners at the hospital, which, I think, in many instances is not well done.
Dr. Spinler: Or if the patient comes back to the pharmacy -- this gets back to our discussion about adherence -- and you see that the patient is no longer taking it, you find out the situation about which they were asked to discontinue it. It could have been a procedure, and it could have been a missed opportunity (to restart it) or that they didn't resume it when the procedure was over.
Slide 10.
Mr. Fanikos: Let me ask about some of the side effects with this newer class of agents. Are there things that we may be unaccustomed to seeing as they come to us with drugs like prasugrel or ticagrelor?
Dr. Dobesh: Yes. With prasugrel being a thienopyridine, much like clopidogrel, the side-effect profiles are extremely similar. The one thing that is a little bit different between prasugrel and clopidogrel is the increased risk for bleeding, and that really goes to the pharmacologic, more potent antiplatelet effect. But I know, Sarah, that you and I have discussed this before with ticagrelor; there are some unique adverse effects.
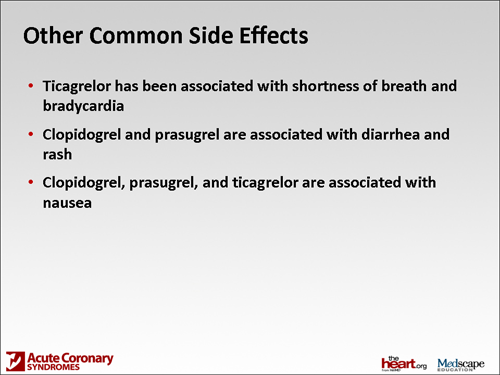
Dr. Spinler: Ticagrelor is an adenosine diphosphate analog that has been associated with shortness of breath, the management of which we don't have any kind of guidance on at this particular point. It may be addressed, for instance, in the product labeling. It certainly was a significant, clinically important side effect that was observed in the platelet trial. Also, bradycardia was seen in the phase 2 trials. Patients who are at risk for bradycardia were excluded from the PLATO trial, so there may also be some issues around potential heart rate monitoring or even heart rate at baseline.
Another side effect of both prasugrel and clopidogrel is nausea, observed with all 3 of the drugs. But diarrhea and rash are associated with clopidogrel and prasugrel. Typically, we as hospital pharmacists don't see that because the hospitalization is so short. They tend to occur somewhere around day 4 and become significant, and that also may cause patients to want to discontinue. I think it's helpful to tell them that up front.
Dr. Dobesh: That happens more than we understand from an inpatient standpoint. The data really say that it happens probably in at least 5%. That's 1 out of 20. When you think of the tens of thousands of patients who take clopidogrel every year or maybe even some prasugrel, that's a lot of rash that's occurring. I agree that that could definitely lead to some discontinuation without healthcare professionals knowing about it.
Mr. Fanikos: The bane, I think, of the existence of every pharmacist who fills prescriptions is alerts for drug interactions. There's been a great deal of controversy over drugs like proton-pump inhibitors (PPIs), which are used universally. What is the current state? Do we have to worry with the newer agents that are out there, with clopidogrel, with prasugrel, with ticagrelor?
Dr. Spinler: He used the term "worry." Paul and I have discussed this. Is it clinically significant or are the data overwhelmingly positive that there is an important drug interaction? No, it's controversial. We talked about this. It's confusing, I think, to practitioners and pharmacists. Whether it's important is still unknown.
Do we worry? Some people worry. There is a warning about the omeprazole-clopidogrel drug interaction, so from the practical aspect, many practitioners would avoid the combination. At our institution we certainly don't give them together, nor do we recommend that patients continue omeprazole as outpatients, primarily because of the product labeling with clopidogrel.
Slide 11.
Dr. Dobesh: Yes. With the newer agents, this whole thing with the PPI drug interaction, I couldn't sit here and say, "Absolutely not," but I'm pretty confident in looking at the evidence. There have been some platelet-aggregation studies with prasugrel, and they really don't show that there's this interaction. It makes sense if you think about the biotransformation of the drug. We've talked already that clopidogrel as a prodrug needs 2 steps.
There are multiple enzymes that take part in this, but 2Y19 is the dominant one in both of those processes; whereas prasugrel is a single step, but it really is not dominated by 2C19. It's more 2B6 or, basically, 3A4 or 5, and 2Y19 plays a much smaller role in the biotransformation. Plus, if one of the enzymes of prasugrel seems to be inhibited for some reason, they can just jump to the next enzyme. The biotransformation with prasugrel: Why does it provide more potent antiplatelet effect? Probably because it's more efficiently converted to the active compound. So, personally, I would not avoid it; there's no warning in the label for prasugrel to avoid PPIs.
The same thing with ticagrelor being a completely different chemical entity: There's no reason to believe, right now at least, that this 2C19 drug interaction possibility would be an issue with ticagrelor. One consideration could be that if someone absolutely has to have a PPI and they can only afford omeprazole, or maybe that's all that's on their plan, maybe they will be using a different antiplatelet agent. What that does from a cost standpoint, we're not really completely sure, but it is an option if you needed to get around that drug interaction. Once again, like Sarah said, I think there's a lot of controversy, and I lean towards the fact that the drug interaction may not be clinically relevant.
Dr. Spinler: One of the interesting studies that was published recently looked at patients who presented with MI and looked back -- I believe it was over the last 6 months -- to see which ones were and were not on PPIs. Patients who were definitely on PPIs had a higher risk for MI, unrelated to being on clopidogrel.
Dr. Dobesh: Yes. In fact, I think there was a 29% increase in risk in those on a PPI without clopidogrel and 29% in those on a PPI with clopidogrel. The clopidogrel seemed to be an innocent bystander in it, so maybe there isn't a drug interaction. Maybe these patients who are on PPIs are sicker patients.
Dr. Spinler: Right. We don't have any large randomized trials that were adequately powered to be able to get to the answer.
Mr. Fanikos: Well, we're running out of time, so I'd like to ask: What do we provide our pharmacist brethren with as a takeaway message for the field and the landscape of antiplatelet therapy over the next year? Paul, do you want to comment on what you think may be important?
Slide 12.
Dr. Dobesh: Yes. It's an exciting time, a lot of changes; therefore, it requires a lot of education. For our pharmacy brethren out there, as we're thinking about this evolving role of new antiplatelet drugs, I don't think we're going to say that any of these new drugs is going to wipe out the existence of the old. We're going to get down into having to know that certain patient populations will get this drug and certain patient populations will get that drug. That makes practice more difficult, but it may make the practice have better efficacy and safety. Although that may be challenging, we've got to dive in and grab the bull by the horns here. From a patient education and a healthcare professional education standpoint, we need to take a leadership role, which is what we're really trained to do.
Dr. Spinler: It's a great opportunity for pharmacists to work with cardiologists and nurse practitioners. I think cardiologists appreciate our drug knowledge and our willingness to help reinforce adherence. Having a set treatment duration plan that's communicated with all of the healthcare providers, perhaps reinforced with cardiac rehab and the reminders that pharmacists can bring -- the duration and maintenance of this is very important. When a patient is undergoing PCI, cardiologists are talking to the patient on the table about taking this medication. It's communicated with them again before they go home, and yet again during discharge counseling, and then hopefully again when they get to the pharmacy. They see all the healthcare workers working together and giving them the same message.
I would urge community pharmacists not to be afraid to communicate with cardiologists. They really want to know how their patients are doing. If there's a problem with the patient not wanting to take it, or looking for the tradeoffs of filling this prescription vs that, or any ways that we could help improve adherence in their patients, they're very interested in hearing from us.
Mr. Fanikos: In summary, what I'm hearing is that there's certainly a role for pharmacists both in the hospital and the community to counsel patients. Pharmacists are in a critical position to provide surveillance for unintended consequences and adverse drug events and outcomes and to communicate back in the form of education and to providers, like cardiologists, to make sure that the outcomes that are being sought are actually reached. Clearly, patient adherence is a big part of that.
I want to thank you both for coming to California and taking time out of your week to talk about this, because I do think it's an exciting time for pharmacists in a lot of different practice settings and there's a lot for us to learn and to provide to our patients who count on our expertise. So thank you. For the audience, thank you for attending this event. We look forward to interacting with you in future educational programs.
Supported by an independent educational grant from AstraZeneca.
This article is a CME/CE certified activity. To earn credit for this activity visit:
http://www.medscape.org/viewarticle/736397
References
- Ferreira-González I, Marsal JR, Ribera A, et al. Background, incidence, and predictors of antiplatelet therapy discontinuation during the first year after drug-eluting stent implantation. Circulation. 2010;122:1017-1025.
- Charlot M, Ahlehoff O, Norgaard ML, et al. Proton-pump inhibitors are associated with increased cardiovascular risk independent of clopidogrel use: a nationwide cohort study. Ann Intern Med. 2010;153:378-386.
Disclaimer
The material presented here does not necessarily reflect the views of Medscape, LLC, or companies that support educational programming on www.medscape.org. These materials may discuss therapeutic products that have not been approved by the US Food and Drug Administration and off-label uses of approved products. A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Medscape Education © 2011 Medscape, LLC



Tidak ada komentar:
Posting Komentar